Innovating Science Small Group Learning: Oxidation-Reduction Reactions
Details
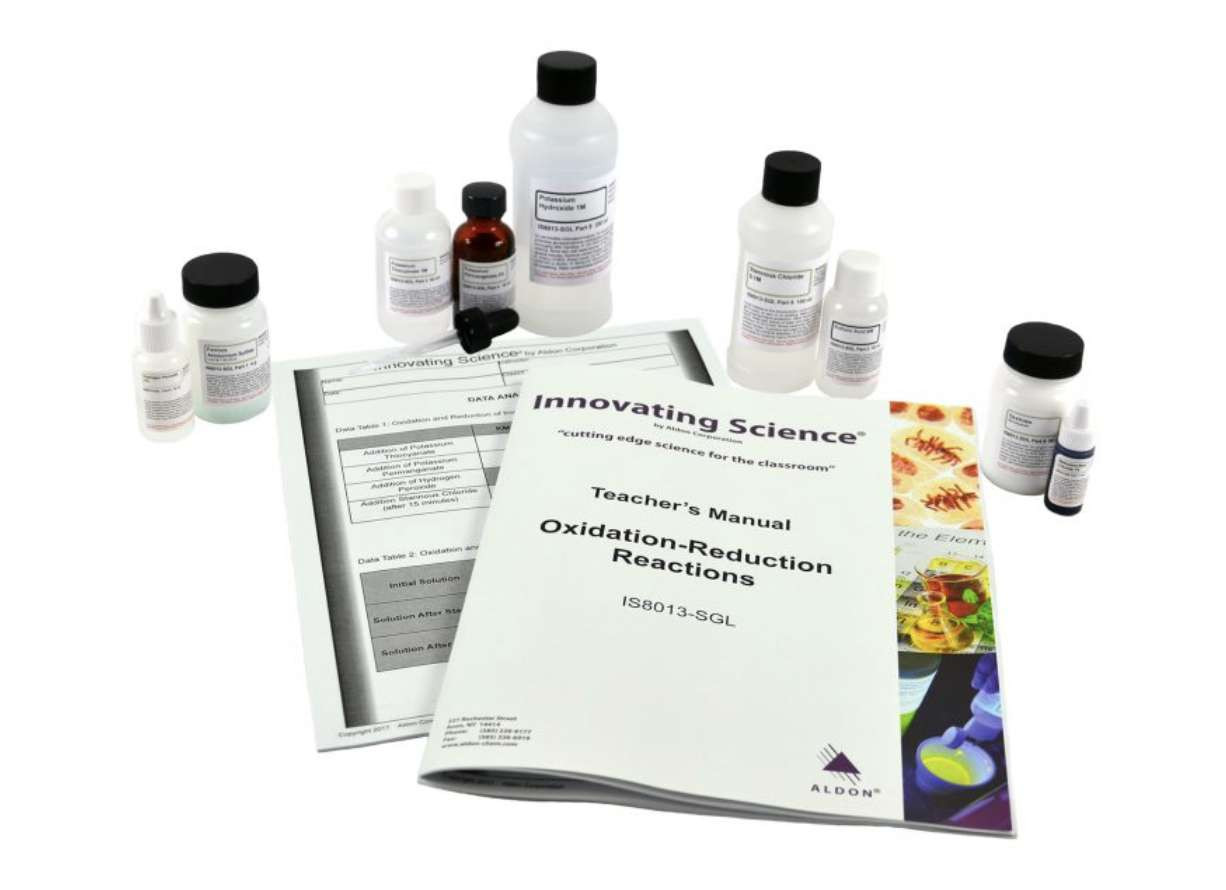
The term oxidation can mean the chemical combination of a substance with oxygen and reduction can be the removal of oxygen from a compound. When oxygen reacts with any other element (except fluorine) it acquires electrons from that element. The element that donated the electrons is said to be reduced. Three experiments will be run where a compound, which is colorless in solution when reduced, is converted to a deeply colored solution when oxidized. The complete balanced reactions for each step should be written showing the transfer of electrons during oxidation and reduction. This kit contains enough material for 5 groups. Teacher's Manual and Student Study Guide copymasters are included.
Kit Includes:
4g Ferrous Ammonium Sulfate
30mL Sulfuric Acid, 6.0M
60mL Potassium Thiocyanate, 1.0M
10mL Potassium Permanganate, 2%
10mL Hydrogen Peroxide, 3%
100mL Stannous Chloride, 0.1M
5mL Methylene Blue, 1%
250mL Potassium Hydroxide, 1.0M
50g Dextrose
DOT Info:
UN1814, Potassium hydroxide, solution, 8, PG II, Ltd Qty
UN2796, Sulfuric acid, 8, II, Ltd Qty

Specifications
Video
Product Attachments
You May Also Like
What Educators are Saying About Geyer
Justin has been nothing but helpful and professional in my requests for these Boeing Defense requirements. He has been able to answer my questions and concerns quickly, and helped in securing the order that I may have made with another supplier had be not been the value-add that he is to your company. I hope his merit raise this FY was "exceeds expectations".
Jesse C...I contacted Geyer via email. I had an email back within minutes!.. A back and forth via email sometimes takes days with other companies and customer service doesn’t happen like this very often. When it does, it’s much appreciated. Brett at Geyer was very polite and accommodating!
John C.The customer service rep went as far as to reach out to the manufacturer of a product to answer my question, and then provided pictures. She really went above and beyond and answered the question thoroughly and gave me exactly the information I was looking for. This entire process was also extremely fast.
Robert C.I called to ask how to submit a Tax Exempt Certificate for my library and spoke to a real person, no automation or asking me to press a number. He was friendly, helpful and got our Tax Exempt status established immediately.
Mischelle F....I don't think I've ever experienced that level of customer service before, and taking it into consideration along with the quality of their products, I would absolutely recommend Geyer to fellow teachers.
Robert S.