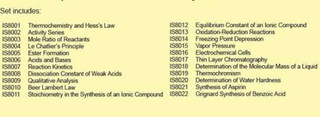
Electrochemical Cells AP Chemistry Test Kit
- Reveal the outcome when reagents accept or donate electrons are arranged so that the electrons can enter or leave the reaction through a metallic conductor.
- Determine why each metal develops a different electrical potential based on its electron configuration.
- How does the voltage a half-cell develop when combined with a hydrogen half-cell?
Details
The tendency of oxidation-reduction reactions is to proceed to an equilibrium state. These reactions occurring in electrochemical cells provide another way for us to express the driving force in chemical reactions. When reagents that accept or donate electrons are arranged so that the electrons can enter or leave the reaction through a metallic conductor, an electrochemical cell is established. A half-cell contains a metal in contact with a solution of its salt. Each metal will develop a different electrical potential based on its electron configuration. The standard reduction potential listed in various references is the voltage that a half-cell develops when combined with a hydrogen half-cell. First, construct a simple chemical battery and determine from the standard reduction potentials what the output of the battery will be (if a voltmeter is available the actual and theoretical voltages can be compared). Second, construct an electrolysis cell and demonstrate how hydrogen and oxygen can be produced from the electrolysis of water.
Kit Includes:
2 x 500mL Cupric Sulfate, 0.5M Solution
1 x 25mL Bromothymol Blue, 0.04% Solution
8 x 500mL Sodium Sulfate, 1M Solution
1 pkg of 15 Copper Metal Strips
1 pkg of 15 Magnesium Metal Strips 5″
1 pkg of 15 Dialysis Tubing Strips 6″
DOT Info:
Small quantity exemption 173.4
THIS PACKAGE CONFORMS TO 49 CFR 173.4 for domestic highway or rail transport only
Specifications
Video
Product Attachments
You May Also Like
What Educators are Saying About Geyer
Justin has been nothing but helpful and professional in my requests for these Boeing Defense requirements. He has been able to answer my questions and concerns quickly, and helped in securing the order that I may have made with another supplier had be not been the value-add that he is to your company. I hope his merit raise this FY was "exceeds expectations".
Jesse C...I contacted Geyer via email. I had an email back within minutes!.. A back and forth via email sometimes takes days with other companies and customer service doesn’t happen like this very often. When it does, it’s much appreciated. Brett at Geyer was very polite and accommodating!
John C.The customer service rep went as far as to reach out to the manufacturer of a product to answer my question, and then provided pictures. She really went above and beyond and answered the question thoroughly and gave me exactly the information I was looking for. This entire process was also extremely fast.
Robert C.I called to ask how to submit a Tax Exempt Certificate for my library and spoke to a real person, no automation or asking me to press a number. He was friendly, helpful and got our Tax Exempt status established immediately.
Mischelle F....I don't think I've ever experienced that level of customer service before, and taking it into consideration along with the quality of their products, I would absolutely recommend Geyer to fellow teachers.
Robert S.